Page 61 - ELT_1st June 2020_VOL 372_Part 5th
P. 61
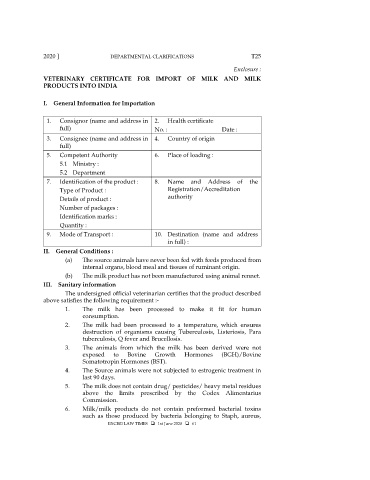
2020 ] DEPARTMENTAL CLARIFICATIONS T25
Enclosure :
VETERINARY CERTIFICATE FOR IMPORT OF MILK AND MILK
PRODUCTS INTO INDIA
I. General Information for Importation
1. Consignor (name and address in 2. Health certificate
full) No. : Date :
3. Consignee (name and address in 4. Country of origin
full)
5. Competent Authority 6. Place of loading :
5.1 Ministry :
5.2 Department
7. Identification of the product : 8. Name and Address of the
Type of Product : Registration/Accreditation
Details of product : authority
Number of packages :
Identification marks :
Quantity :
9. Mode of Transport : 10. Destination (name and address
in full) :
II. General Conditions :
(a) The source animals have never been fed with feeds produced from
internal organs, blood meal and tissues of ruminant origin.
(b) The milk product has not been manufactured using animal rennet.
III. Sanitary information
The undersigned official veterinarian certifies that the product described
above satisfies the following requirement :-
1. The milk has been processed to make it fit for human
consumption.
2. The milk had been processed to a temperature, which ensures
destruction of organisms causing Tuberculosis, Listeriosis, Para
tuberculosis, Q fever and Brucellosis.
3. The animals from which the milk has been derived were not
exposed to Bovine Growth Hormones (BGH)/Bovine
Somatotropin Hormones (BST).
4. The Source animals were not subjected to estrogenic treatment in
last 90 days.
5. The milk does not contain drug/ pesticides/ heavy metal residues
above the limits prescribed by the Codex Alimentarius
Commission.
6. Milk/milk products do not contain preformed bacterial toxins
such as those produced by bacteria belonging to Staph, aureus,
EXCISE LAW TIMES 1st June 2020 61